HOCl vs NaOCl: Which Disinfectant Wins?
Introduction: Our Clean Obsession
We’re Shandong Shine, and we live for clean. Not just regular clean—hospital-grade, germ-smashing, virus-killing clean. That’s why we specialize in both Shine Hypochlorous Acid Generators and Shine Sodium Hypochlorite Generators. You’ve probably asked yourself at some point: Is HOCl better than NaOCl? Or, what even are these two weird names?
Well, you're in the right place. Let’s break down the battle between hypochlorous acid (HOCl) and sodium hypochlorite (NaOCl). Two big names. Two powerful disinfectants. One final verdict.

The Basics: Meet HOCl and NaOCl
HOCl: The Friendly Assassin
· Chemical Name: Hypochlorous Acid
· Formula: HOCl
· pH Range: ~5.0 to 6.5
· Charge: Neutral
· Type: Weak acid
· Formed by: Electrolyzing saltwater
HOCl is a gentle killer. Sounds scary? It shouldn’t. It’s actually produced by white blood cells in your body to fight off pathogens. How cool is that?
NaOCl: The Classic Heavyweight
· Chemical Name: Sodium Hypochlorite
· Formula: NaOCl
· pH Range: ~10 to 12
· Charge: Negative (OCl⁻ ion)
· Type: Strong alkaline
· Found in: Bleach
NaOCl is what most people know as household bleach. It’s been around forever and gets the job done—just not always in the gentlest way.

Key Differences: HOCl vs NaOCl
pH Levels: Acidic vs Alkaline
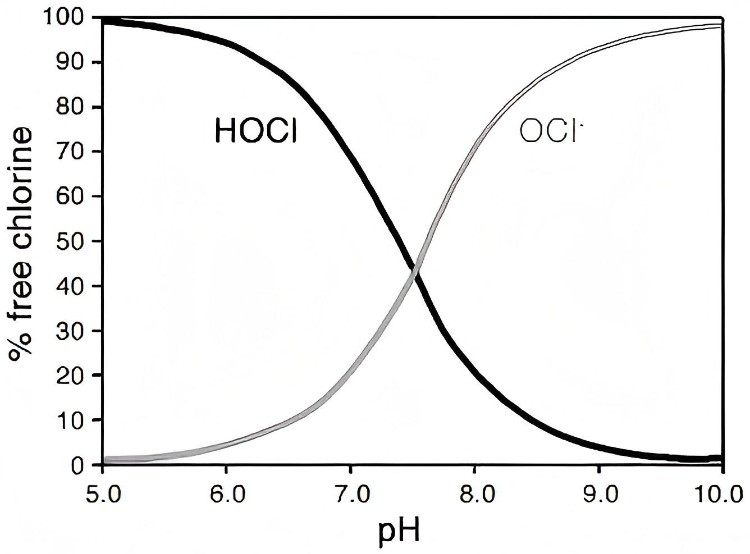
· HOCl operates at a neutral to slightly acidic pH (5–6.5).
· NaOCl needs a high pH (~11–12) to remain stable.
Here’s the kicker—HOCl’s pH range makes it far less corrosive. We can apply it without damaging surfaces or irritating our skin. That’s a win in our book.
Charge and Penetration
· HOCl is neutral, allowing it to pass through cell walls with ease.
· NaOCl carries a negative charge, which limits its effectiveness on bacteria with similar charges.
So even though both are strong oxidizers, HOCl has the upper hand in infiltration and speed.
Kill Bacteria Like a Pro
Both HOCl and NaOCl can kill bacteria, fungi, and viruses. But HOCl acts faster.
· HOCl: Kills microbes in seconds
· NaOCl: Takes minutes
Think about that. When seconds count—like in food processing or hospitals—you want speed. And that’s where HOCl shines.
Environmental and Human Safety
What We Breathe Matters
· HOCl = No chlorine gas
· NaOCl = Risk of releasing chlorine gas at lower pH
Chlorine gas is dangerous. It irritates lungs, eyes, and the environment. Why take that risk?
Skin Contact and Eye Safety
· HOCl = Gentle enough for wound care
· NaOCl = Causes irritation, even burns
We’ve sprayed HOCl on fruits, baby toys, and our hands. Try doing that with bleach and see how fast people start coughing.

Application Areas: Who Uses What?
Where HOCl Rules
1. Food industry: For its non-toxic profile
2. Healthcare: Used in wound irrigation
3. Childcare centers: Safe for high-touch areas
4. Dental practices: Kills bacteria without harming tissue
5. Farming: Especially in livestock and poultry care
Where NaOCl Still Reigns
1. Water treatment plants
2. Industrial disinfection (high-volume use)
4. Laundry and sanitation
Even though it's older tech, sodium hypochlorite still works wonders in massive systems where budget and volume matter more than finesse.

Why We Make Both at Shine
Let’s be real. Not every job needs the finesse of HOCl. Some situations need the raw power of NaOCl. That’s why we offer both.
· Shine Hypochlorous Acid Generator: Delivers pure HOCl, perfect for health-focused industries
· Shine Sodium Hypochlorite Generator: Built for bulk operations and water systems
We didn’t choose sides—we built both. Because every surface, every industry, and every need is different.
Fun Chemistry: HOCl Formation & Breakdown
HOCl is born when chlorine dissolves in water:
Cl₂ + H₂O → HOCl + HCl
At higher pH levels, HOCl dissociates into hypochlorite ions (OCl⁻):
HOCl ⇌ H⁺ + OCl⁻
So here’s a twist: even bleach relies on HOCl when it works! But the higher pH prevents it from being as effective.
Want effective disinfection? Keep the pH slightly acidic. That’s when hypochlorous acid HOCl is in its strongest form.
Pros and Cons Breakdown
HOCl: Pros ✅
· Safe for skin
· Neutral pH
· Fast kill time
· No toxic residue
· Effective at low concentrations
· Kills viruses, bacteria, fungi
HOCl: Cons ❌
· Less stable—must be used fresh
· Sensitive to sunlight
NaOCl: Pros ✅
· Long shelf life
· Inexpensive
· Easy to store in large volumes
· Strong oxidizer
NaOCl: Cons ❌
· Harsh on skin and eyes
· Corrosive on metals
· Releases chlorine gas in acidic environments
· Slower disinfection rate

Shine's Take: What We Recommend
We’ve worked with thousands of facilities across food, medical, and agriculture. And here’s what we’ve seen:
· If human contact, airborne misting, or organic material is involved—HOCl wins
· If pipe systems, municipal water, or cost-driven applications—NaOCl still has a place
But when we need power plus safety, HOCl takes the gold every time.
The Future of Disinfection Is Clear
Let’s not sugarcoat it—chlorine-based disinfectants still dominate. But consumers are demanding cleaner, safer, and smarter solutions. HOCl fits the bill.
So whether you're treating water, sanitizing a hospital room, or cleaning a baby’s pacifier—we’ve got you covered. From acid water to hypochlorite ions, we’re here to help you disinfect smarter.
