Reaction of Hypochlorous Acid and Calcium Hydroxide
Hypochlorous acid (HOCl) plays an important duty in several cleansing and disinfecting procedures. But did you recognize that when it reacts with calcium hydroxide, a whole new collection of chemical interactions take place?
In this post, we'll discover just how this reaction unravels, what it implies for cleanliness, and why it's important in generating hypochlorous acid making use of day-to-day products like table salt and deep sea.
We'll likewise touch on the duty of HOCl generators, the threats of chlorine bleach, and how this response can kill microbial pathogens without resorting to toxic chemicals.

What is Hypochlorous Acid (HOCl)?
At its core, hypochlorous acid is an effective disinfectant. You could already know it as the energetic component in some sanitizers and cleansing remedies. HOCl is normally created by our bodies' immune systems to eliminate off infections. In industrial applications, it's typically created making use of a HOCl generator machine.
When hypochlorous acid is produced from table salt and deep sea, it's essentially a more secure, much less hazardous choice to chlorine bleach, though it shares some similar top qualities in terms of its ability to disinfect and eliminate microbial pathogens.
The Role of Calcium Hydroxide in the Reaction
Now, let's dive into the heart of this post-- the reaction between calcium hydroxide and hypochlorous acid. Calcium hydroxide, frequently called slaked lime, is a solid base, and when it enters contact with acids, it undergoes a neutralization response.
The response in between calcium hydroxide and hypochlorous acid types calcium chloride (CaCl ₂), water (H ₂ O), and hypochlorite ions (ClO ⁻). This process is necessary because it develops an extra secure type of chlorine, which can be utilized in numerous applications, consisting of disinfection and sanitation.
Exactly How Does Hypochlorous Acid React with Calcium Hydroxide?
Right here's a streamlined variation of the chain reaction:
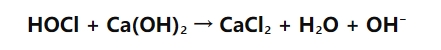
This reaction showcases just how hypochlorous acid connects with calcium hydroxide. The calcium hydroxide reduces the effects of the hypochlorous acid, causing the manufacturing of calcium chloride, which is a salt, and water. The essential takeaway is that it minimizes the acidity of the remedy while still leaving behind reliable chlorine-based disinfectants like hypochlorite ions.
Why This Reaction is Important
1. Developing Hypochlorous Acid Safely
You do not require harmful chemicals or factory-made materials to produce hypochlorous acid. By utilizing table salt and saltwater with a HOCl generator machine, you can generate this effective disinfectant in the convenience of your very own area. This chemical reaction demonstrates how an easy base like calcium hydroxide can assist develop a less harmful, much more stable disinfecting solution.
2. A Better Alternative to Chlorine Bleach
Standard chlorine bleach is a widely known anti-bacterial, however it features its disadvantages. The reaction of HOCl with calcium hydroxide supplies a more secure, a lot more efficient solution. While chlorine bleach can be harsh and toxic, the results of hypochlorous acid manufacturing are more secure and less most likely to irritate the skin or generate dangerous fumes.
The Science Behind Generating Hypochlorous Acid
When salt water is electrolyzed in a HOCl generator, hypochlorous acid is created with the procedure of electrolysis. Electrolysis entails passing an electrical current with the option, which triggers sodium chloride (table salt) to break down and develop both sodium hypochlorite (NaOCl) and hypochlorous acid (HOCl).
How Hypochlorous Acid Kills Microbial Pathogens
Among one of the most effective features of hypochlorous acid is its capability to eliminate microbial pathogens. The acidic nature of HOCl disrupts the cell membrane layers of bacteria and viruses, making them inadequate. Whether you're utilizing it in medical care settings, food safety, or cleanliness, hypochlorous acid properly reduces the effects of hazardous pathogens without considering toxic chemicals.
Is This Process Safe?
When produced appropriately, hypochlorous acid is much safer than other anti-bacterials like chlorine bleach. Generating hypochlorous acid through a HOCl generator machine ensures that the process is controlled, with marginal danger to human health. Plus, it avoids the rough chemicals generally utilized in traditional sanitizers and disinfectants.
The Environmental Impact of Calcium Hydroxide and Hypochlorous Acid
Among the major benefits of this reaction is its minimal environmental effect. The byproducts-- water, calcium chloride, and hypochlorite ions-- are non-toxic and can be safely taken care of. Unlike toxic chemicals like chlorine bleach, this response doesn't generate dangerous deposits or fumes, making it a much better alternative for both wellness and the environment.
The Benefits of Using a HOCl Generator
Below are some key factors to use a HOCl generator machine:
Safe and safe: Unlike bleach or other commercial chemicals, hypochlorous acid is non-toxic.
Cost-efficient: Once you have a HOCl generator, you can produce hypochlorous acid as needed, conserving cash in the long-term.
Powerful anti-bacterial: HOCl is extremely effective against a vast array of microorganisms, consisting of bacteria, infections, and fungis.
Applications of Hypochlorous Acid
The versatility of hypochlorous acid is vast, including applications in:
Food security: It's utilized to wash fruit and vegetables and sanitize food surface areas.
Medical care settings: It's a favored anti-bacterial due to its safe nature.
Water therapy: HOCl aids purify water and get rid of dangerous microbes.
Home cleaning: Hypochlorous acid can be utilized as a cleaner for numerous surfaces.
The Drawbacks of Using Chlorine Bleach
While chlorine bleach has been a common anti-bacterial for years, it features several drawbacks:
Poisoning: It can aggravate the skin, eyes, and breathing system.
Extreme chemicals: Chlorine bleach has harmful compounds that can contaminate the environment.
Minimal performance: It does not work as effectively on specific types of virus compared to HOCl.
Verdict: A Cleaner, Safer Future
The response of hypochlorous acid and calcium hydroxide uses a look right into the future of more secure, extra reliable cleanliness. By creating hypochlorous acid from salt and water, we can generate a potent anti-bacterial that's less harmful than chlorine bleach, while still taking on microbial pathogens head-on.
Whether you make use of a HOCl generator machine or a DIY technique, this response demonstrates how science can supply us with much better choices to toxic chemicals.
As we continue to seek environmentally-friendly and safe services, recognizing the chemistry behind responses like this will help us create much safer rooms for everyone.
