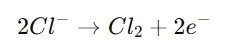Reaction Name For Electrolysis Of Saline Hypochlorous Acid
The electrolysis of saline solution is a crucial procedure for generating hypochlorous acid (HOCl). This process integrates science and practicality, leading to a powerful disinfectant. Whether you're utilizing an HOCl generator machine or doing the electrolysis at home, comprehending the response and the ingredients included is crucial to optimizing the outcomes. Let's simplify detailed.
What is Electrolysis?
Electrolysis is a chemical process that utilizes electrical power to drive a non-spontaneous reaction. When it comes to saline solution (salt water), electrolysis breaks down the compound right into its elements. In this situation, sodium chloride (NaCl) dissociates right into salt (Na) and chloride ions (Cl-). These ions after that take part in a chain of reactions that causes the generation of hypochlorous acid.
Key Components in Electrolysis
1. Salt (NaCl): The primary ingredient, frequently in the form of sodium chloride, is liquified in water to create a saline solution.
2. Water (H ₂ O): The medium where electrolysis happens. When salt is dissolved, it creates a liquid solution.
3. Electrical power: The power supply that drives the electrolysis response. A consistent electric cost is essential to generate hypochlorous acid.
The Reaction: Breaking It Down
In an electrolyzed water supply, the sodium chloride solution undergoes an electric existing. Right here's the core of the response:
At the Anode (Positive Electrode):
Chloride ions (Cl-) are oxidized, generating chlorine gas (Cl ₂).
Reaction:
This chlorine gas dissolves in the water, developing hypochlorous acid (HOCl).
At the Cathode (Negative Electrode):
Water molecules go through reduction to form hydrogen gas (H ₂) and hydroxide ions (OH ⁻).
Reaction:
The hydroxide ions add to the development of sodium hydroxide (NaOH).
What Happens Next?
When chlorine gas and sodium hydroxide exist, they respond to create sodium hypochlorite (NaOCl), a kind of bleach, and hypochlorous acid (HOCl). These 2 compounds are responsible for the decontaminating power of electrolysis-treated water. The balance in between HOCl and NaOCl is what determines the strength of the solution.
· Chlorine gas dissolves in the water to create hypochlorous acid (HOCl), which is a really powerful disinfectant.
· The electric fee and liquid solution aid balance the formation of sodium hydroxide and hypochlorous acid.
The Role of pH while doing so
The pH of the solution directly impacts the outcome of electrolysis. At a reduced pH (acidic problems), hypochlorous acid is more common, making it an effective antimicrobial. In contrast, at greater pH (alkaline problems), sodium hypochlorite is the dominant compound, which is less efficient for sanitation but still useful for cleansing.
Just How HOCl Impacts the Body
Hypochlorous acid is essential for the immune system, especially for white blood cells. These cells produce HOCl to combat off infections. The capacity to duplicate this procedure making use of an HOCl generator machine in decontaminating environments is a significant benefit.
Electrolysis in Everyday Life: Salt and Water
You don't need an industrial-scale machine to generate hypochlorous acid. As a matter of fact, you can create a basic electrolysis setup utilizing salt and water at home. By dissolving salt (NaCl) in water, you produce a saline solution that can be dealt with making use of electricity to generate chlorine-based anti-bacterials.
The Benefits of Electrolyzed Water
Electrolyzed water, whether utilized for creating hypochlorous acid or other chlorine solutions, has numerous applications:
· Sanitation: Effective in killing bacteria and viruses, particularly in healthcare setups.
· Cleansing: Safe for surfaces in kitchens, shower rooms, and various other high-traffic locations.
· Water Treatment: Used in swimming pools and day spas for disinfection.
Safety Precautions in Electrolysis
While producing hypochlorous acid is typically safe, you need to handle it with treatment:
1. Ventilation: Chlorine gas is toxic in high concentrations. Guarantee proper air flow.
2. Handling Chemicals: When dealing with chlorine solutions and sodium hydroxide, usage handwear covers and prevent direct contact with skin.
3. Display pH Levels: An excessively acidic or basic solution might create inflammation or damages to surfaces.
Why Use a HOCl Generator Machine?
A HOCl generator machine makes the procedure of producing hypochlorous acid a lot more reliable. These equipments permit regular production of high-grade hypochlorous acid by managing variables like electric cost, pH levels, and salt concentration. It's a hassle-free and safe way to produce disinfectants as needed, without the requirement for harsh chemicals.
From Salt Water to Powerful Disinfectant: The Process Explained.
1. Prepare the Solution: Mix salt (NaCl) with water to form an aqueous solution.
2. Power Up: Use a power supply to generate the electrical cost required for the electrolysis process.
3. Electrolysis Reaction: Chlorine gas is created at the anode, while sodium hydroxide and hypochlorous acid form at the cathode.
4. End product: You currently have hypochlorous acid (HOCl), which can be used for sanitization.
Applications of Electrolyzed Water
Cleansing and Disinfection: Electrolyzed water is a safe alternative to conventional cleaning solutions.
· Agriculture: Used for sterilizing fruits, vegetables, and equipment.
· Medical: Used to sanitize surface areas in healthcare facilities and facilities.
· Industrial: Applied in factories for keeping hygiene standards.
Common Questions About Electrolysis of Saline
1. Can I utilize salt and vinegar for electrolysis? Yes, vinegar can be made use of as a source of acid for the electrolysis process, however deep sea is much more usual.
2. Is hypochlorous acid safe for skin? HOCl is naturally generated by our white blood cells and is safe in percentages for cleansing wounds or sterilizing skin. Nevertheless, high concentrations can be annoying.
3. What is the difference in between sodium hypochlorite and hypochlorous acid? Sodium hypochlorite is a less powerful anti-bacterial contrasted to hypochlorous acid. HOCl is more efficient at killing bacteria and viruses at lower pH degrees.
The Future of Electrolyzed Water Technology
As innovation developments, we may see even more portable, efficient single-cell systems for electrolysis, making hypochlorous acid generation a lot more easily accessible. From home cleaners to commercial sanitizers, the future of electrolyzed water looks intense.
Conclusion
The electrolysis of saline solution is a remarkable chemical procedure with wide-reaching applications. From basic salt water to powerful hypochlorous acid, the process relies upon basic principles of electric fee and chain reaction. Whether you're utilizing an HOCl generator machine in your home or working in an industrial setting, the power of electrolyzed water is undeniable.