The Fascinating Reaction Between Hypochlorous Acid and Water

When we talk about hypochlorous acid, it usually sounds like a chemistry lesson straight out of senior high school. Yet the reality is, this chemical is at the core of some attractive amazing processes-- whether it's in cleansing, sanitation, and even your body's immune system.
So, what takes place when hypochlorous acid responds with water? Allow's dive into the reaction in between hypochlorous acid and water, exploring its chemical nature, its uses, and why it's so essential for human health and hygiene.
What Is Hypochlorous Acid?
Prior to we go into the specifics, allow's specify hypochlorous acid, or HOCl. It's a weak acid created when chlorine dissolves in water. We commonly read about its benefits in sanitation and disinfecting, but it's also normally present in the body, specifically in white blood cells, where it plays an essential duty in combating infections.
HOCl is usually generated via electrolyzed water, a process where salt water goes through electrolysis to produce both hypochlorous acid and hypochlorite ions.
The Chemistry of Hypochlorous Acid and Water
At its core, the reaction in between hypochlorous acid and water is reasonably uncomplicated yet interesting. When HOCl is introduced right into water, it partially dissociates into hypochlorite ions (ClO-) and hydrogen ions (H+). Below's the formula to offer you a clearer image:
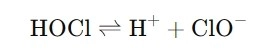
In this response, the hypochlorous acid breaks down into a mix of acidic and oxidizing components, which are essential to its sanitizing power. The balance between HOCl and hypochlorite ions depends on aspects like pH, temperature, and focus. When the pH is reduced, extra hypochlorous acid kinds, giving the solution more powerful sanitizing residential properties.
Why Is This Reaction Important?
You may ask yourself why this response matters. Well, it's essential for both industrial usages and biological procedures. In the world of hygiene, the ability of HOCl to work as an effective anti-bacterial comes from its ability to break down bacteria, infections, and various other harmful microorganisms. It's no wonder that many cleansing solutions, consisting of those produced by HOCl generators, count on this reaction to offer a quickly, effective disinfecting solution.
In your body, the response plays a lot more important duty. When white blood cells battle infection, they produce hypochlorous acid as part of the immune reaction. This natural chemical functions as a solid oxidizer, breaking down unsafe intruders without triggering damages to surrounding cells.
What Is Electrolyzed Water?
Electrolyzed water is water that has been treated with an electric current, causing a reaction in between salt (sodium chloride) and water molecules. This procedure produces a mix of hypochlorous acid and hypochlorite ions, which makes the water very effective at disinfecting surfaces, killing bacteria, and also cleansing water. The charm of electrolyzed water is that it's a safe, eco-friendly choice to typical chemical anti-bacterials.
The tools utilized to create electrolyzed water is called a HOCl Generator. These generators convert deep sea into hypochlorous acid and sodium hypochlorite by applying an electrical charge, generating a solution that is capable of killing virus and cleaning surfaces. It's a sustainable, safe alternative for services, medical facilities, and homes alike.
Just How Does Hypochlorous Acid Benefit Our Health?
The response between hypochlorous acid and water does more than just sterilize-- it has straight benefits for our health. As an example, in our white blood cells, HOCl is used to counteract harmful pathogens in the body. When created in large amounts, it can aid your body combat bacteria, viruses, and even fungis, which is why it's usually used in medical setups for injury cleansing and infection control.
The Role of Hypochlorite Ions in Cleaning
On the flip side, hypochlorite ions (ClO-) are likewise generated when hypochlorous acid connects with water. These ions are recognized for their solid bleaching and decontaminating residential properties. Sodium hypochlorite, a typical anti-bacterial discovered in bleach, is basically a solution of hypochlorite ions in water. The hypochlorite ions work by breaking down the cell walls of bacteria and various other bacteria, making them safe.
Exactly How Salt Water Creates Hypochlorous Acid
The procedure of generating hypochlorous acid utilizing salt water is what makes HOCl generators so effective. The electrolysis of salt water (sodium chloride, NaCl) produces a chemical reaction that produces both HOCl and sodium hydroxide (NaOH). The sodium hydroxide, which is alkaline, assists to support the hypochlorous acid, making it more reliable for cleansing and disinfection.
The response goes like this:
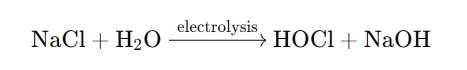
This process generates a highly effective solution for cleaning, sterilizing, and disinfecting different surface areas. The capability to generate hypochlorous acid on-site, as opposed to relying upon pre-made chemicals, is a game-changer for several industries.
Practical Uses of Hypochlorous Acid in Cleaning
When you make use of a HOCl generator, you're tapping into an effective disinfectant that's efficient at reducing the effects of a wide range of microorganisms. Whether it's in healthcare facilities, institutions, kitchen areas, or homes, hypochlorous acid is highly efficient versus bacteria, viruses, and even mold and mildew.
Some typical applications consist of:
Surface Area Disinfection: Cleaning kitchen counters, floorings, and high-touch surfaces.
Food Safety: Rinsing vegetables and fruits to eliminate unsafe bacteria.
Air Purification: Eliminating airborne microorganisms and odors.
Water Treatment: Purifying drinking water and pool.
The Connection Between Sodium Hypochlorite and Hypochlorous Acid
Sodium hypochlorite (NaOCl), generally called bleach, is a close loved one of hypochlorous acid. While both chemicals work anti-bacterials, there's a vital difference in their chemical structure. Sodium hypochlorite is usually generated by including chlorine gas to a solution of sodium hydroxide. It's less reliable than hypochlorous acid in its pure type but is still extensively made use of for cleansing functions.
Hypochlorous Acid in Industry
In industry, HOCl is very demanded for its flexibility. Electrolyzed water solutions are increasingly popular as they are safer, environmentally friendly, and non-toxic. For sectors such as food processing, water filtration, and healthcare, the capacity to generate hypochlorous acid on-site with a HOCl generator uses a cost-efficient solution to preserving high requirements of hygiene and safety and security.
Is Hypochlorous Acid Safe?
Yes! One of the standout advantages of hypochlorous acid is its security. Unlike many standard anti-bacterials, it is safe to human beings and animals, making it an ideal choice for both commercial and residential cleansing. When used effectively, it doesn't position any kind of considerable health and wellness risks, which is a massive plus in an age where people are coming to be extra conscious of the chemicals they use.
Final thought: Why HOCl Is a Game Changer
When we look at the reaction between hypochlorous acid and water, it's simple to see why this substance is so useful. Whether you're utilizing it to decontaminate surface areas with an HOCl generator or depending on your immune system to eliminate infection, HOCl plays a critical function in keeping us safe and healthy and balanced. The capability to generate hypochlorous acid from deep sea is an innovative growth, providing us a sustainable, effective solution for cleanliness and health. So, next time you make use of hypochlorous acid, bear in mind the chemistry behind it-- and the substantial impact it carries both our day-to-day lives and our health and wellness.
