The Hidden Power of Hypochlorite Ion
What is Hypochlorite Ion?
The hypochlorite ion (ClO⁻) is a strong oxidizing agent. We usually meet it in bleach solution or sodium hypochlorite NaOCl. This ion acts as the active ingredient that wipes out bacteria and viruses fast.
I remember the first time I poured household bleach into a stained shirt. Watching it whiten clothes felt like magic. Later, I learned that behind that magic sits a tiny ion that carries enormous power.
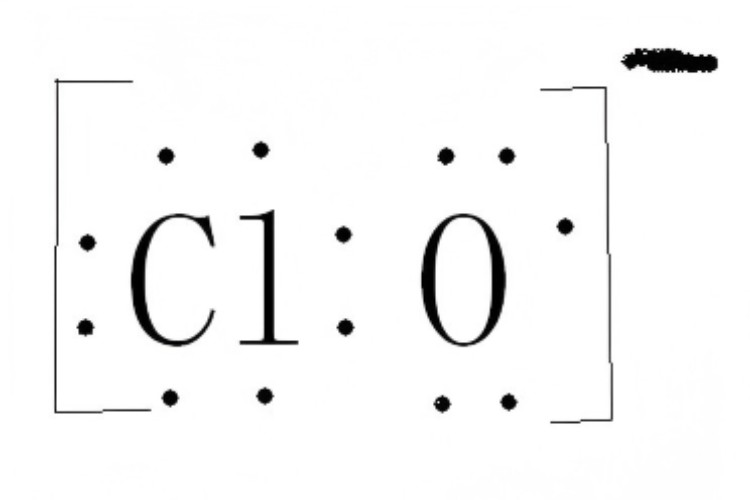
How Hypochlorite Ion Works
So, what makes it powerful?
It attacks cell walls of microbes.
It reacts with proteins and enzymes.
It breaks down bacteria and viruses at the root.
When added in small amounts to water treatment systems, hypochlorite ion reduces the risk of disease outbreaks.
Killing Bacteria in Water
Cities rely on a solution of sodium hypochlorite for disinfection. The aqueous solutions keep our tap water clean and drinkable. Without it, we’d face countless waterborne illnesses.
Fighting Viruses
Hypochlorous acid HOCl and hypochlorite combine to create a one-two punch. They neutralize dangerous pathogens faster than most disinfectants.
Sources of Hypochlorite Ion
Where does this ion come from? Let’s break it down.
Sodium Hypochlorite NaOCl
This is the star of the show. It forms when chlorine reacts with sodium hydroxide. The result is an aqueous bleach solution used across industries.
Calcium Hypochlorite
Often sold as pool tablets, calcium hypochlorite serves as another source of hypochlorite ion. It keeps swimming pools clear and safe.
Commercial Bleaching Agents
From concentrated hypochlorites in factories to commercial bleaching powders, the ion drives cleaning processes worldwide.
Everyday Uses of Hypochlorite Ion
We use hypochlorite ion more often than we realize.
Household Bleach
At home, it removes stains, deodorizes, and brightens. That bottle under the sink hides a lot of chemistry.
Swimming Pools
When you dive into cool blue water, hypochlorite works quietly. It kills microbes, balances hygiene, and makes swimming enjoyable.
Commercial Bleaching
Industries count on concentrated hypochlorites for whitening textiles and sanitizing equipment. It’s tough on dirt yet adaptable to different needs.
The Chemistry of Hypochlorite Ion
Let’s peek at its structure.
Formula: ClO⁻
Charge: -1
State: Usually found in aqueous solutions
Partner: Often paired with sodium chloride in bleach
When dissolved, hypochlorite ion exists alongside hypochlorous acid HOCl. This mix drives the strong disinfection action we depend on.
Hypochlorite and Safety Concerns
Now here comes the twist. While hypochlorite ion is a hero, it can turn dangerous.
Inhaling chlorine gas from improper mixing causes harm.
Concentrated hypochlorites burn skin and eyes.
Improper storage lowers effectiveness.
We must handle bleach solutions carefully. Respecting it keeps us safe.
Safe Practices
Always dilute before use.
Store away from heat and sunlight.
Never mix with acids or ammonia.
I once made the mistake of mixing cleaners. The chlorine gas that formed taught me a hard lesson. Safety first, always.
Hypochlorite in Water Treatment
When it comes to water treatment, hypochlorite ion stands unmatched.
It kills bacteria and viruses quickly.
It maintains water safety during storage.
It prevents algae growth in reservoirs.
Communities across the globe rely on this ion to provide clean water. Without it, disease would spread like wildfire.
Industrial Hypochlorite Generation
In industrial settings, companies produce bleach onsite. Using a Sodium Hypochlorite Generator, they avoid shipping hazardous chemicals. This also ensures fresh production, which keeps the active ingredient strong.
Why We Rely on Hypochlorite Ion
Here’s why this ion holds a special place in our lives:
It keeps water safe to drink.
It whitens clothes and brightens fabrics.
It supports food safety through disinfection.
It helps reduce the risk of infection in hospitals.
It works across both household and industrial systems.
Without it, our everyday lives would look very different.
Quick Facts About Hypochlorite Ion ClO
Household bleach contains 3–6% sodium hypochlorite solutions.
Swimming pools often use calcium hypochlorite tablets.
Industrial bleach may reach 12–15% concentrated hypochlorites.
Small amounts are enough to sanitize large water volumes.
The active ingredient loses power if stored too long.
Final Thoughts
When we ask “What is Hypochlorite Ion?” the answer is simple but powerful. It’s the chemical warrior that fights bacteria and viruses, whitens clothes, and keeps water safe. Sure, we must watch out for hazards like inhaling chlorine gas or mishandling concentrated hypochlorites. But used wisely, this ion protects our health and our daily lives.
I see it not just as a chemical, but as a hidden guardian. Every time I drink clean water or swim in a safe pool, I silently thank hypochlorite ion for doing the dirty work.
